A lot can be learned from history, what to do, what not to do and what to do a little differently. We looked to the genius of an older method of refining, which virtually disappeared from use due to the introduction of the simple Cyanidation process, aka MacArthur-Forrest process patented 1887. We built upon the Chloride method, made it simple, safer, more practical and most importantly environmentally friendly.
History
History
Newbery-Vautin chlorination process cir. 1887
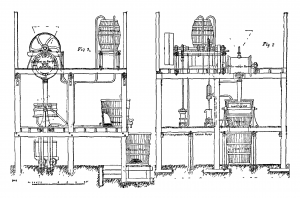
The Newbery-Vautin chlorination process was a process to extract gold from its ore using chlorination developed by James Cosmo Newbery and Claude Vautin. The process of extracting gold from ores by absorption of the precious metal in chlorine gas, from which it is reduced to a metallic state, is not a very new discovery. It was first introduced by Karl Friedrich Plattner around 1848, and at that time promised to revolutionize the processes for gold extraction. By degrees it was found that only a very clever chemist could work this process with practically perfect results, for many reasons. Lime and magnesia might be contained in the quartz, and would be attacked by the chlorine. These consume the reagents without producing any results, earthy particles would settle and surround the small gold and prevent chlorination, then lead and zinc or other metals in combination with the gold would also be absorbed by the chlorine; or, again, from some locally chemical peculiarity in the water or the ore. Later inventors Henderson, Clark, De Lacy, Mears, and Deacon, all introduced improvements, or what were claimed to be improvements, on Plattner, but these chiefly failed because they did not cover every particular variety of case which gold extraction presented. Therefore, where delicate chemical operations were necessary for success it is not to be wondered why processes requiring such care and uncommon knowledge were not greatly in favor at the time.
"Trilogy, the modern Newbery-Vautin process"
The main advantage of the Chlorination Process to dissolve gold is the high stability of the solutions and their low oxidation potential (Qi et al. 1991; Ahgelidis et al. 1995).
Trilogy Refining uses a patented proprietary method which uses electricity, common salt, water, and swimming pool acid which are common products available at any home improvement store. The hydro-metallurgical process re-uses the solution over and over again, the Gold and (PGM) metals are captured on the resin material, any heavy metals dissolved in the solution are removed and captured in additional resin columns and the resulting solution is either reused or discarded as reclaimed water. Our exclusive and patented recovery resin technology has virtually eliminated the early problems associated with the use of chlorine and the problems of waste bi-products.
We use proven technologies with modern methods
Why Chlorine
We choose Chlorine based upon the solubility of gold in aqueous solutions of the chemicals named. Lixiviation by chlorine solutions are what we are particularly interested in. The relative dissolving powers of the three chemicals in percent solutions, with each solution at ambient temperature, over the specific time periods of the following:
- Chlorine in 6 hours dissolves 57% of gold
- Bromine in 6 hours dissolves 49% of gold
- Cyanide in 6 hours dissolves <6% of gold
All three processes seem to occupy a distinctive field of their own, but also encroach upon each other’s territory. In such cases the choice of the process should depend upon which will extract the greatest percentage of gold at the least cost with the lowest ecological impact.
Our Chemistry
Chlorination was used two centuries ago before the use of cyanidation. Chlorine was first used to recover gold from residues from amalgamation. Later, in the 1880’s Chlorination was used in big mining operations in the American and Australian gold fields. At the present time unfortunately chlorination for gold recovery is used on extremely small scale where gold is a minor constituent with other precious metals. Gold dissolves in aqueous chloride solution to form gold+ and gold3+ chloride complexes.
Au + 2Cl– = AuCl2 – +e
Au + 4Cl– = AuCl4 – + 3e
Silver and lead can form insoluble chlorides in chloride media. This is important because insoluble products can reduce the solubility of gold due to the formation of an insoluble layer. Nevertheless, Passivation can occur to any important degree when the silver and lead content of the gold alloy is more than approximately 18%.
In addition telluride, sulfide ores are soluble in acid chloride media when there is an oxidant such as chlorine, and dissolve to form compounds of gold.. The behavior of carbonates are interesting because the decomposition of these minerals help to recover gold because there is a better exposition of locked gold.
